GUEST BLOG: Will the 21st Century Cures Act 2.0 Deliver Innovation and Health Breakthroughs for Post-Infection Diseases?
By Emily Taylor, Solve M.E. Director of Advocacy and Community Relations
Five years ago, the 21st Century Cures Act (Cures Act) was signed into law making national headlines, launching new health initiatives, and spearheading a new chapter in government scientific investment. With robust funding, multiple government agencies rolled out programs designed to accelerate medical product development and bring new innovations and advances to patients who need them faster and more efficiently.
This year, the original champions of the Cures Act, Representatives Fred Upton (R-MI) and Diana DeGette (D-DO), are revisiting their efforts and crafting new legislation to take the next steps to build on the Cures Act. Earlier this year, they released a 21st Century Cures Act 2.0 discussion draft.
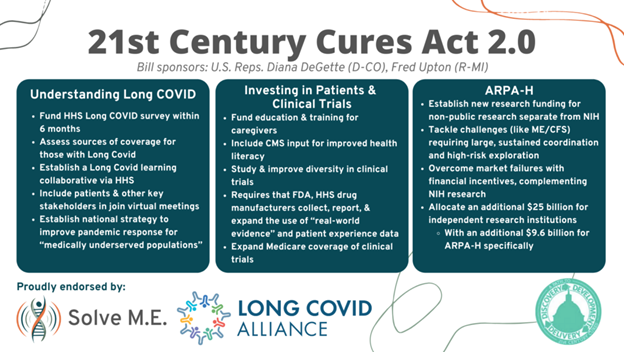
What’s really hope-inspiring is the way the proposal takes a science-forward and evidence-based approach to treatments and that they focused significant attention on long-COVID. For example, the first section, “Further Understanding the Implications of Long COVID,” delivers federal leadership regarding the gaps in health care coverage for millions of COVID-19 long haulers who have fallen through the cracks of our traditional health care and safety net systems.
Next, the proposed Long COVID learning collaborative will fill another crucial void — diagnostics and therapeutics for Long COVID. While the National Institutes of Health’s (NIH) $1.15 billion RECOVER Initiative has begun the quest in “basic science,” the real need is for patients to GET IDENTIFIED and GET WELL. Our nation cannot afford to sacrifice an estimated 3.2 million people to lifelong chronic illness. Reps. Upton and DeGette’s proposal is the first major congressional step to address this public health crisis since Reps. Beyer and Bergman’s COVID-19 Long Haulers Act.
Another innovation is quietly peppered throughout the bill and offers big hopes for post-infection illness patients. Three words: PATIENT. EXPERIENCE. DATA. Seemingly simple, this will revolutionize the research landscape for anyone who’s ever been told by their doctor, “I can’t find anything wrong with you,” when they know that simply isn’t the case. In a systematic commitment, the Cures 2.0 Act enables federal agencies to utilize information drawn from patient experience, real-world evidence, and patient-reported outcomes. While not every agency will evolve right away, Cures 2.0 sets into motion the critical first steps towards a revolutionary transformation in research: a world where patient voices are central.
The act would also create ARPA-H, Advanced Research Projects Agency for Health. This new agency’s mission would be to “speed transformational innovation in health research and speed application and implementation of health breakthroughs” by funding projects that could:
- Tackle bold challenges requiring large scale sustained coordination;
- Create new capabilities (e.g., technologies, data resources, disease models);
- Support high-risk exploration that could establish entirely new paradigms;
- Overcome market failures through critical solutions, including financial incentives; and
- Complement NIH’s existing research portfolio and mission and the private sector’s research initiatives.
Dr. Francis Collins, Director of the NIH, wrote about ARPA-H’s potential in ARPA-H: Accelerating biomedical breakthroughs. We’re enthusiastic about the capability of this initiative to tackle the big challenges, and we’re ready to fight for post-infection disease to be one of the inaugural projects if ARPA-H is implemented next year.
Those concerned with post-infection diseases should especially pay attention to ARPA-H. One of the biggest challenges in funding treatment trials and hypothesis-generating research is that NIH doesn’t fund it. ARPA-H could provide a research home for the public health projects left behind, deemed not profitable enough by investors.
While all of this is exciting, the biggest impact is yet to come! Nothing can beat cold hard cash in the race for research, and Cures 2.0 delivers in this area. The final provision of the bill, the “Research Investment to Spark the Economy” or RISE Act, allocates $25 billion to independent research institutions, public laboratories, and universities throughout the country. Not only does this investment combat the COVID-19 disruption to our R&D, but also kickstarts America’s competitive edge internationally, where many believe we’re falling behind.
There are many exciting reasons to be optimistic about the future of America’s biomedical infrastructure, but only if we give it the vision it deserves. Cures 2.0 could be the way to a better, healthier, future.
Solve M.E. is a member of the National Health Council. For more information on membership, click here.