FDA Regulations


Ensuring FDA effectively regulates 21st Century Products
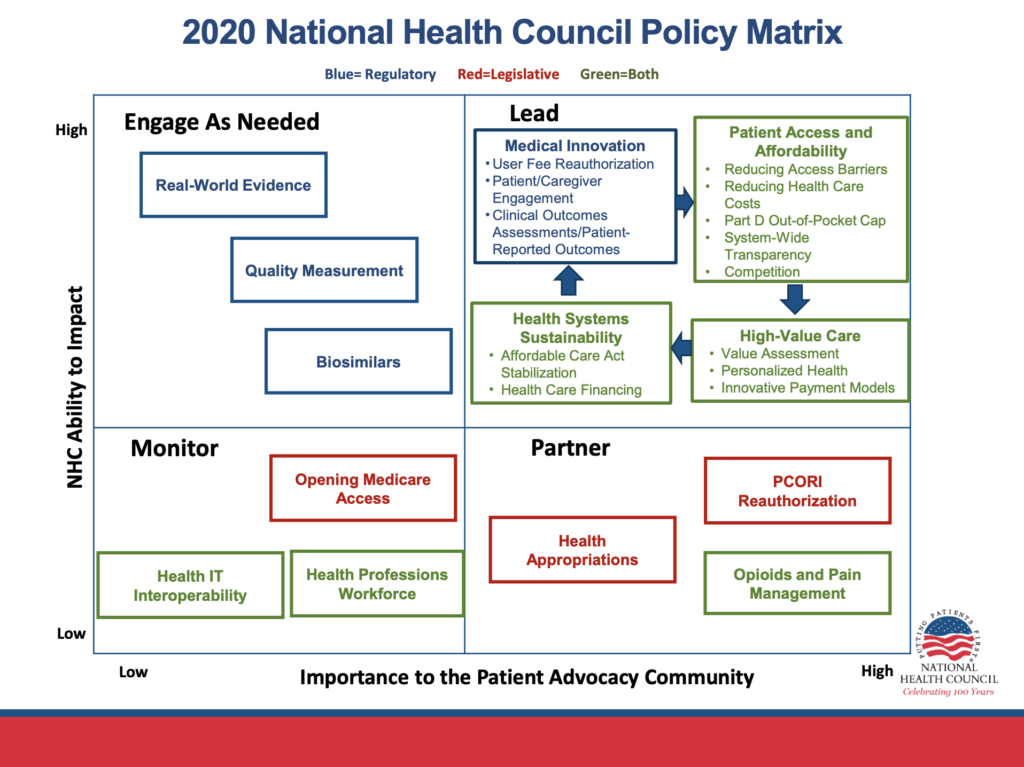
The NHC supports the Food and Drug Administration’s (FDA) role of ensuring safety and efficacy of medical products. Through policies such as the user fee reauthorizations and 21st Century Cures Act, we advocate for FDA to have the staff expertise and tools they need to keep pace with rapidly evolving science.
Meetings & Events
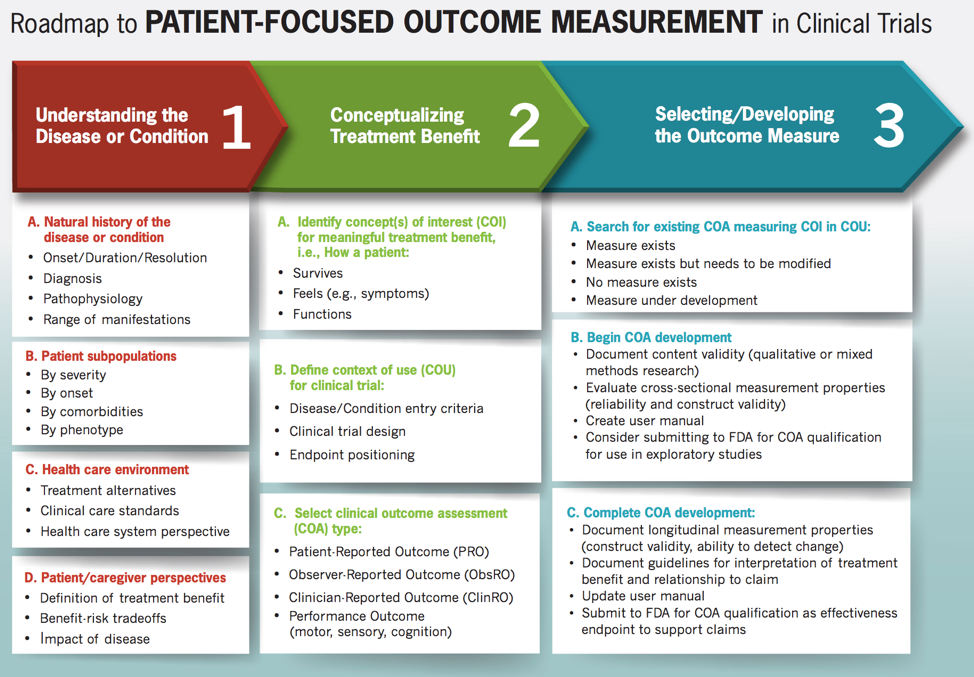
Webinar: Perspectives on the FDA’s 2nd Patient-Focused Drug Development Guidance
12/13/2019 1:00 pm, Webinar Read MorePatient-Focused Medical Product Development Webinar Series
The NHC’s Patient-Focused Medical Product Development (PFMPD) series covers a broad range of subjects, including patient preference research, patient engagement in clinical trial design, as well as medical device development. The NHC seeks to ensure that its membership is prepared to engage in conversations on all aspects of PFMPD.
Blogs
News
No resources found.