Background
Introduction
The National Health Council (NHC) and its partners have undertaken the creation of a Blueprint for developing “patient-centered core impact sets,” or PC-CIS, to address inconsistencies between what patients* report as important to them versus much of the information that is routinely collected in research and care.
A PC-CIS is a patient-derived and patient-prioritized list of the most important impacts a disease and/or its treatments have on a patient’s health and daily life and that of their family and caregivers. *
Intentionally broad and inclusive, the term “impacts” refers to the things patients tell us are important about the way a disease and/or its treatment affects their daily lives (and that of their family and caregivers). Impacts include short- and long-term health outcomes and any other related implications (e.g., mental health effects, caregiver/family stresses, economic burden, work and career loss, etc.).
*Throughout the Blueprint, the term “patient” can be used to include carers and families as appropriate.
About the PC-CIS Blueprint
PC-CIS Blueprint Process Overview
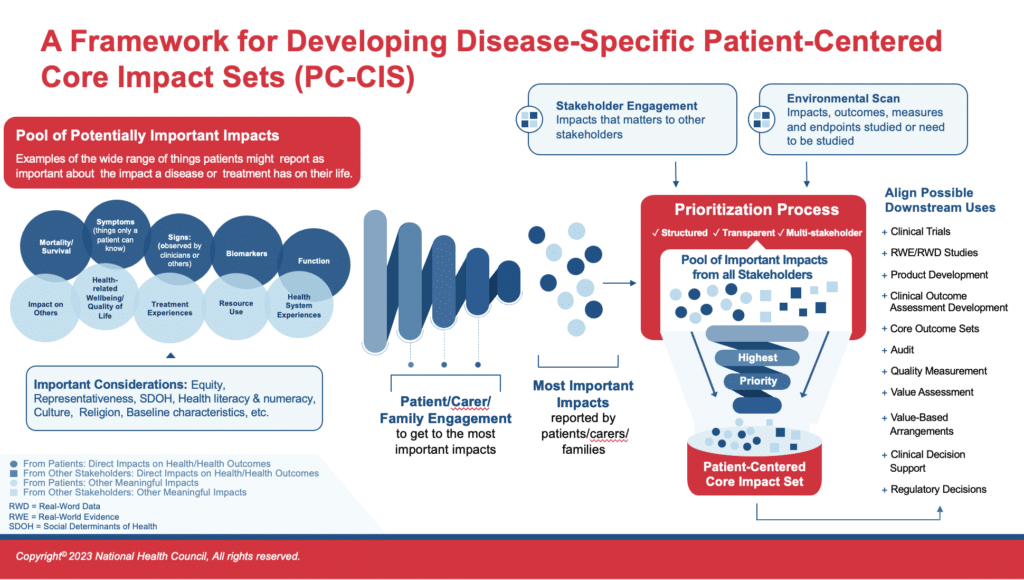
As reflected in this graphic, the PC-CIS Blueprint differs markedly from past efforts to create other core sets. While other initiatives begin with an evaluation of the existing research, often an environmental scan that includes a review of the literature, the PC-CIS Blueprint starts with insights and data generated directly by and from patients. Learnings from literature and other stakeholders are important, but come later in the process, helping to shape the impact set but never supplanting the impacts patients report as most important.
A key consideration for the Blueprint is efficiency. That is, it emphasizes drawing from existing materials to inform the work without replacing the patient-prioritized information. This could include such things as drawing from past, qualitative research efforts and reports on data derived directly from patients.
Viewing PC-CIS as a Necessary Precursor Step for Downstream Uses
The focus of a PC-CIS is the identification and prioritization of core, patient-reported and -prioritized impacts. The core can inform a multitude of downstream decisions and uses such as the development of measures or tools. However, the focus is not to zero in on measurement tools upfront. The identification, adaptation, or creation of measures, for example in the clinical outcome assessment (COA) environment is envisioned as downstream – after the PC-CIS is created. The PC-CIS is a precursor that informs measure creation or selection. Thus, the creation of tools is not covered here or under the resources and guidance provided within the Blueprint. The Blueprint only guides the process for getting to the PC-CIS. It provides examples of what downstream uses might be, such as COA measure development, but it does not delve into the details and methods for those other efforts.
For a quick reference guide about the steps in and use of the Blueprint, please see the Blueprint Process Overview and Checklist. As described there, the first phase is Planning, which includes three activities intended to occur in tandem. Beginning with the second phase, Gathering Data, each step is then intended to be sequential.
A hallmark of the Blueprint and the PC-CIS that will result, is the order of the data gathering and prioritizing steps. Patient data is gathered and prioritized first, so that it is unencumbered by other stakeholder data, existing/past research, and related biases. Organizing the data collection and prioritization in this way ensures that impacts most important to patients end up in the final impact set, including those that may have historically not been collected or reviewed.
An Intentional Process to Support Patient Centricity
It is important to stop here and comment on the intentional order of data collection outlined in this Blueprint and noted in the PC-CIS Process Overview figure. You will see that gathering data on impacts from patients and prioritizing that patient-reported data precedes the environment scan and the collection of data from other stakeholders. This order is intentional to reduce bias. The rationale is to keep the data collected from and prioritized by patients free from the potential influence of past or current efforts that may inadvertently or intentionally influenced what the most important impacts should be (from a view that is not the patients or family caregivers such as a clinician).
For example, suppose the creation of a PC-CIS begins with an environmental scan of the literature, producing a list of impacts assumed to be most important to patients because of the frequency with which they appear in clinical-trial literature. However, the patients who participated in the trials were never asked what was important to them. The trial outcomes and endpoints reflect the views of the clinical researchers, what they viewed as important or assumed was important to patients. If the literature-derived list is provided to patients (for comment or prioritization), it can bias patient responses in a leading way and produce a list of impacts that do not reflect what patients would have said more organically. The core list produced would not be patient centered.
For this reason, two separate data gathering efforts are described in the Blueprint, one from patients and one from other sources (other stakeholders and an environmental scan). All impact data is then combined in the second, overall prioritization process. Two important considerations happen in this second prioritization: 1. All evidence on all important impacts is provided from all sources to all participants, and 2. The patient-prioritized impacts are protected from elimination.
Understanding the Steps: Sequence and Skipping
Must the data gathering be done sequentially? |
• The two data gathering steps could be done in tandem if it can be assured that the patient data collection is not biased by data from the other sources. This is likely very difficult to do. The prioritization processes should be kept separate to avoid influencing patients’ prioritization. |
Can Steps be Skipped? |
• We strongly discourage skipping any steps in the PC-CIS Blueprint. However, we understand that for some organizations this can be a daunting process that will have to be completed incrementally over time as resources are identified. Thus, we recommend that interim or draft PC-CIS products be made available and used as each phase of the process is completed. These interim products are useful and valuable to stakeholders as a team works its way through the Blueprint. These interim work products are identified in the Blueprint Checklist. |
PC-CIS Blueprint: What, Who, and Why?

What?
A Step-by-Step Process
The PC-CIS Blueprint offers a step-by-step process for making use of and best understanding the knowledge patients and families have about the impacts of disease and treatment. Only patients can tell us about their lived experiences and what is most important to them. But not all patients, even within the same disease community, will have the same experiences and priorities. Using sound, representative patient-engagement methods can help to provide the foundation for identifying and prioritizing the impacts of most concern, and the core impacts most important to patients.
This PC-CIS Blueprint is just that — a guide or a map to navigate the process. However, the PC-CIS Blueprint document itself is disease and population agnostic, meaning the users will need to adapt and modify as needed to best fit their unique needs and goals, populations and subpopulations, and disease nuances. It is intended to be a flexible guide and not overly prescriptive. We recognize there are gaps in knowledge and in those cases, we have attempted to fill those gaps with practical advice and considerations in this evolving field.

Who?
Patient Organizations and Others in the Patient Community
The PC-CIS Blueprint is intended as a resource for patient organizations and others interested in developing a disease- or population-specific PC-CIS. It will help guide them through the phases of developing a PC-CIS but is not a substitute for partnering with experienced researchers and other experts as needed. We have attempted to glean best practices based on existing work and past experiences and provide citations and links to those useful resources for PC-CIS-development teams to turn to.

Why?
A PC-CIS Capturing the Patient Voice for Downstream Uses
The creation of a disease- or population-specific PC-CIS is valuable for a wide range of downstream purposes, including, but not limited to, planning for and conducting clinical research, selecting or developing Clinical Outcome Assessment (COA)tools, revising or creating clinical practice guidelines, developing methods for tracking and measuring quality of care, value and health technology assessment (HTA) projects and analyses, and many other activities or initiatives in the health care and research ecosystem.
How can PC-CIS be used?
While the creation of a PC-CIS is intended to be use agnostic, the potential applications for various PC-CIS are numerous. The table below provides nine examples to help illustrate this wide utility, but this collection of examples is not meant to be exhaustive or prescriptive. Please note: These are hypothetical examples.
Clinical Trials | Real-World Evidence/Real-World Data Studies | Product Development |
Systematic Review | Quality Measurement | Value Assessment |
Value-Based Arrangements | Clinical Decision Support | Regulatory Decisions |
It is also important to understand that how a PC-CIS is used will likely depend on the community or activity in question; not all impacts will always be relevant in all cases. For example, there may be regional differences such that the impacts most important in a US patient cohort differ slightly from those in a European cohort. Conversely, stage of disease in a community may dictate which items from a PC-CIS are most central to a given task. The PC-CIS is meant to be the full collection of prioritized impacts, from which those relevant to a particular need can be selected. The rationale for not addressing an impact should be made clear to avoid actual or the appearance of “cherry picking” certain impacts.
PC-CIS as a Catalyst for Culture Change
As articulated in the published article about Patient-Centered Core Impact Sets (see The Patient), there are many reasons why creating PC-CIS is worthwhile and beneficial to research and healthcare innovation. By starting with data from patients, analyzed and applied in partnership with patients, PC-CIS capture the full complement of disease and treatment impacts most important to patients. Conventional methods for defining patient-important outcomes have been self-limiting, often built on existing research that may or may not have included patients as partners. Often, what patients deem most important may be considered difficult to measure and for that reason may be ignored. The value of PC-CIS comes not only from the resulting list, but from the creation process itself.
Despite the many benefits of PC-CIS, there is likely to be some push back or resistance to PC-CIS given the significant paradigm shift it represents. PC-CIS proponents might encounter difficulty getting other stakeholders to buy into the Blueprint or the PC-CIS concept. It can be difficult to get people and organizations to change to a new way of thinking, especially when they are invested in the processes they have in place and are used to their own way of doing things. This issue is not new when trying to motivate change and it is often referred to as trying to “change the culture” of an organization or field. Proponents of PC-CIS might experience some resistance for a variety of reasons, from concern about cost outlays or that it will extend tight timelines, to some feeling their expertise or authority is threatened. As in any type of culture change, it may not be possible to push for complete and total adoption right from the start, but the Blueprint equips users with multiples tools and ideas for how to make as much progress as possible.
Making the Value Case, Facilitating Acceptance
Given the potential for resistance, we understand that the patient community and their partners may need to “sell” the concept of PC-CIS to others to gain its acceptance and engender collaboration. Here, we provide several suggestions on how proponents can discuss the value proposition for PC-CIS:
- PC-CIS are necessary precursors to achieving more universal alignment around what is important to patients.
PC-CIS are a mechanism to inform future work in many areas to ensure that work is patient centered. PC-CIS aligns varied, often unrelated, activities toward addressing the same set of core impacts. For example, clinical trials, comparative-effectiveness economic analyses, and health care quality measurement would all be aligned on the same set of patient-derived and prioritized impacts. Today, this is not happening, but PC-CIS can be a step in that direction.
- PC-CIS can improve efficiency and reduce burden in collecting patient experience data from patients and families.
Patient experience data is the primary source of data, the pipeline into a PC-CIS. Thus, a PC-CIS serves as the first “stop” to identify relevant impacts important to a patient community, across any conceivable purpose. A disease- or population-specific PC-CIS, provides a clear starting place, reducing the need for redundant primary data collection. New data collection can inform a PC-CIS and help build new knowledge, but an existing PC-CIS provides the foundation for alignment across all types of work (e.g., COA development or study endpoint selection).
- PC-CIS development is intended to be led and driven by the patient community.
The PC-CIS initiative is different. To ensure the patient voice is heard, it is intended that PC-CIS development be led by the patient community in collaboration with other stakeholders. Instead of the patients being invited to someone else’s table, a patient group or other patient community organization is doing the inviting of experts and others to its table.
- PC-CIS do not replace, displace, or overshadow critical and foundational efforts that have come before it.
Rather, PC-CIS serve in a symbiotic relationship, building on existing research and other pivotal work, while informing current and future efforts to capture and measure what is most important to patients. For example, a PC-CIS would not replace a current core outcome set. It informs new core outcome set development and can be used to align and adapt existing sets to be sure the patient voice is reflected.
- A PC-CIS is not a list of measures, but does inform measure selection and development, and adds efficiencies.
Measures should reflect what patients tell us is important. Rather than undergo qualitative research to determine if and which measures are needed, researchers can turn to existing PC-CIS and build on that knowledge in their measure development.
- PC-CIS can unlock efficiencies across research and healthcare innovation.
Many patients experience more than one disease. Core impacts that are common across disease states or subpopulations could not only alleviate burden for patient communities, researchers, clinicians, and others, but help to augment our ability to understand the experiences of patients with more than one condition, a frequent and confounding challenge. If we understand the common impacts that cut across diseases, we might be able to develop tools to capture those impacts, reducing redundancies in systems and burden on patients.