FDA’s Roadmap to Patient-Focused Outcome Measurement in Clinical Trials
04/18/2019
By Elisabeth M. Oehrlein, PhD, MS, Senior Director, Research and Programs
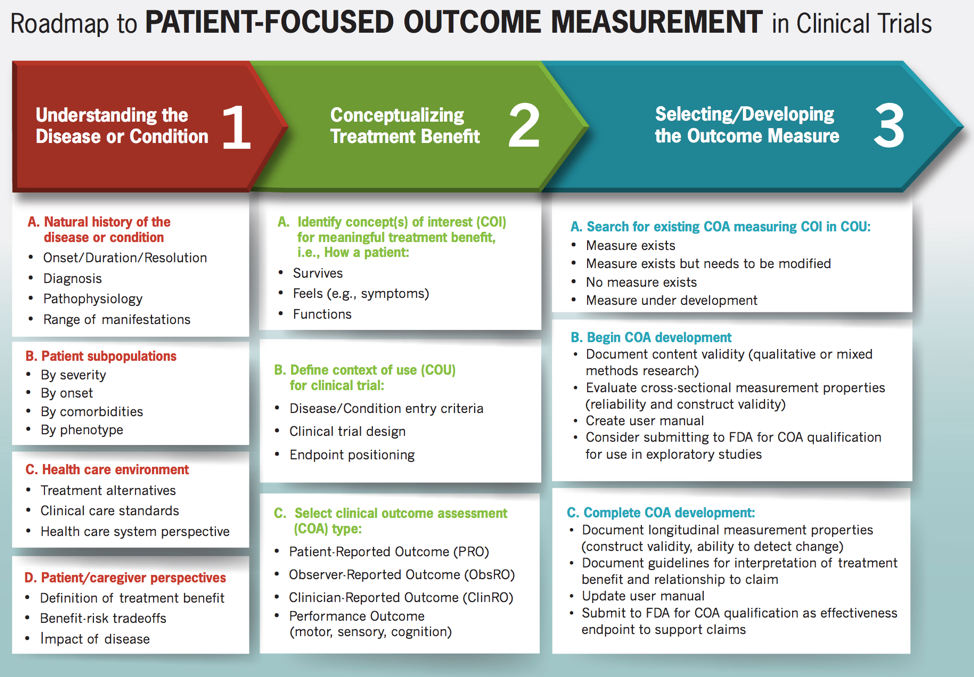
Patient-centered research and care focuses on outcomes that are most important to patients, which often are about how they feel or function. Measures often need be developed to capture meaningful data on these kinds of outcomes in clinical trials for drug approval. The second webinar in our Clinical Outcome Assessment (COA) series introduces participants to the Food and Drug Administration’s (FDA) Roadmap to Patient-Focused Outcome Measurement in Clinical Trials. The Roadmap (in the picture below) was developed by the FDA to help researchers understand and then develop new or adapt existing tools to measure these important outcomes. This webinar features Laurie Burke, RPh, MPH, Founder, LORA Group LLC and Former Director of FDA’s Study Endpoints and Labeling Development group, where she led FDA’s COA team and developed the Roadmap.
The FDA emphasizes that gathering the necessary information to develop or adapt a COA measure can be an extremely lengthy process. Industry sponsors should engage early with COA experts, patients, caretakers, and with the FDA as well to develop a COA that will generate reliable, valid, and interpretable data.
The Roadmap has three steps:
Part 1: Understanding the Disease or Condition
Researchers must first develop a complete and comprehensive understanding of the disease. That incudes understanding the patient’s view on living with their illness.
Part 2: Conceptualizing Treatment Benefit
Clinical benefit is defined as the effect of an intervention on how an individual feels, functions, or survives. It is critical to define clinical benefit from patient and caregiver perspectives. An important step in part 2 is identifying which type of COA measure is most appropriate given the “thing” (concept of interest) being measured. Recall that not all patient-centered outcomes are reported by patients. Watch the webinar of this topic.
Part 3: Selecting/Developing the Outcome Measure
Once developers have adequately researched the first two sections, then they can move onto the third column. Armed with information on which outcomes are of greatest interest to patients, researchers can then identify if an appropriate measure already exists and has been validated in the population it is intended to be used in, or if a new one needs to be developed from scratch. Sometimes an existing measure can be modified and validated in the population to save time and resources.
How can patients be involved in the COA development process?
Patient groups can help advance patient-centered drug development by the having information needed in column one at the ready. That can save considerable time and effort to speed the process and ensure the patient voice is accurately captured.
Note: Forthcoming FDA guidance on PFDD may result in modifications to the Roadmap.
Watch the webinar about FDA’s Roadmap here.