Core Outcome Sets – What are They?
08/05/2019
By Aimee Lee Russell, Associate, Programs
On Monday, July 15, Donna Messner, PhD, CEO and President of the Center for Medical Technology Policy (CMTP) introduced core outcome sets and described why they are important to patients. This was the ninth webinar in the NHC’s Clinical Outcome Assessment (COA) series.
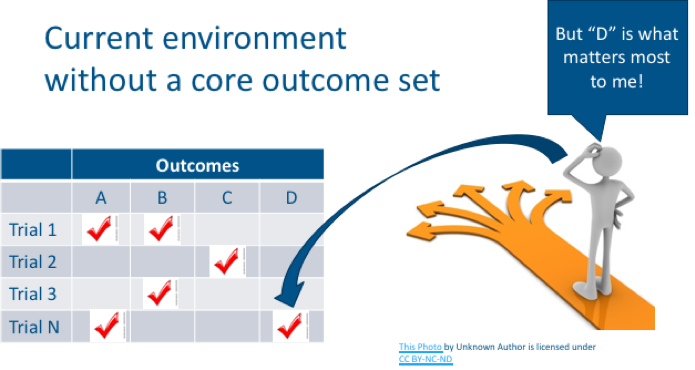
Dr. Messner described how in an ideal clinical-research scenario, study results would measure and report all of the same outcomes and produce comparable evidence for a treatment. Unfortunately, that is not how clinical research happens. Outcomes that are studied for one class of treatments can vary from study to study. Only some outcomes can be compared or combined across studies. While certain studies might include outcomes that patients care about, other studies might measure things that patients don’t care as much about.
In core outcome set development, stakeholders come together to identify which outcomes are the most important and should be measured consistently across all studies in a given disease. Of course, researchers are welcome to also measure outcomes beyond those included in a core set, but at a minimum, they should measure the outcomes within the agreed upon core set. It’s essential that patients participate in core outcome set development to ensure that outcomes included in the core set reflect the things that patients care about.
Core outcome sets are an agreed upon, standardized set of measures that should be gathered and reported as a minimum in all clinical research in specific areas of health or health care. – COMET
Dr. Messner described a specific example in development of a core outcome set: the coreHEM. In coreHEM, CMTP and their partners engaged a multitude of stakeholders – including patients and patient advocates. Please listen to Donna Messner’s presentation for more information about core outcome sets.