PFMPD Webinar Series: Rare-Disease Listening Sessions with NORD and the FDA
By Aimee Lee Russell, Associate, Programs
This blog highlights the first webinar in our new Patient-Focused Medical Product Development (PFMPD) webinar series. This series builds upon our Clinical Outcome Assessment (COA) series, which we hosted between November 2018-December 2019. The new series will continue to introduce COA-related topics, but also will cover a broader range of subjects, including patient preference research, patient engagement in clinical trial design, as well as medical device development.
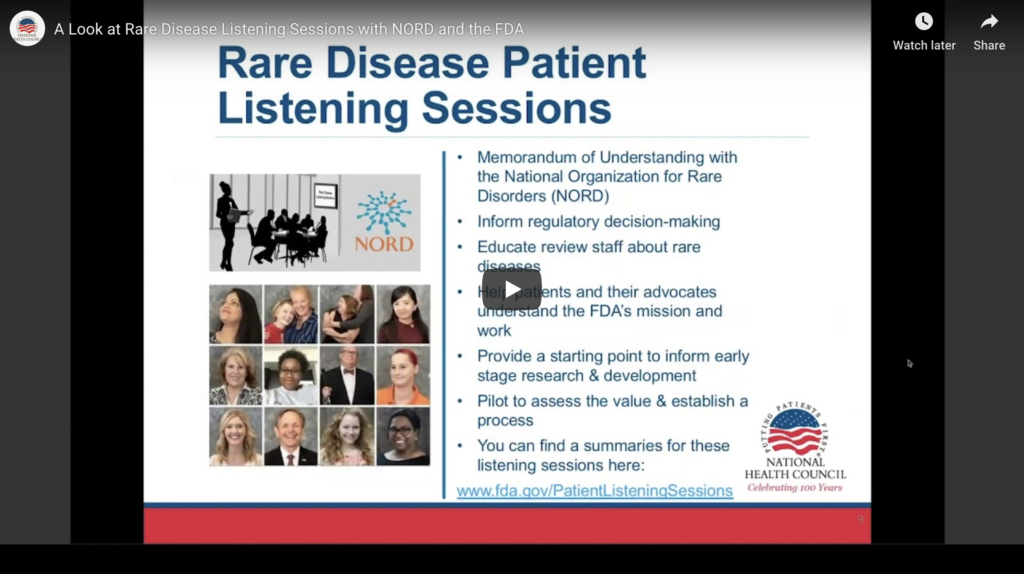
On Friday, January 31, Debbie Drell, Director of Membership at the National Organization for Rare Diseases (NORD) and Andrea Furia-Helms, MPH, Director, Patient Affairs Staff at the U.S. Food and Drug Administration (FDA) discussed their organization’s collaboration on Rare Disease Patient Listening Sessions.
The Rare Disease Listening Sessions are informal, non-advisory, closed discussions between FDA staff and members of the rare-disease patient community. The table below highlights more specifics on the Listening Sessions. The Listening Sessions are meant to focus on patient experience with a disease and are not meant to be representative of an entire community.
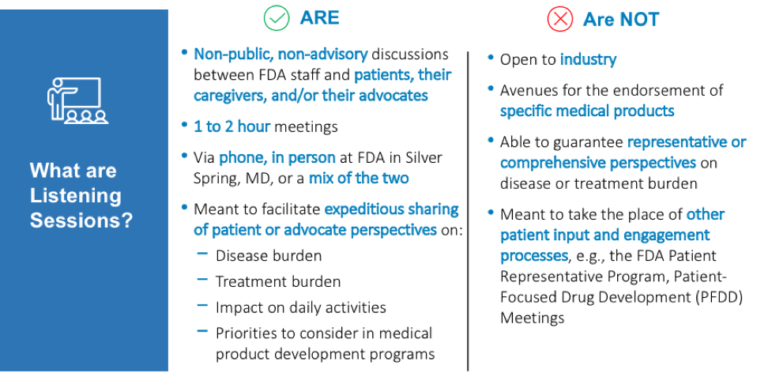
There are two types of Listening Sessions, FDA-requested sessions where a specific set of questions are asked of a particular patient population or sub-population and Patient-requested when a patient community wants to share their experiences and perspectives with the FDA. Members of the patient community can request a listening session through this website.
Ms. Drell described NORD’s role in coordinating between FDA and patients through a memorandum of understanding between the two organizations. In addition to recruiting participants, NORD also helps participants prepare for the meetings to ensure they are comfortable speaking during the session.
For more information, please click here to watch the full webinar.
Links to summaries from listening-sessions-to-date are copied below.
This list is accurate as of February 12, 2020. For the most up-to-date list, please visit the FDA’s Patient Listening Sessions website.
FDA-Requested Listening Session Summaries
- December 2, 2019 – Gastroparesis (summary coming soon)
- November 13, 2019 – Childhood Cerebral Adrenal Leukodystrophy (CCALD) (summary coming soon)
- October 17, 2019 – Sanfilippo Syndrome – pediatric (PDF – 219KB)
- May 13, 2019 – Sanfilippo Syndrome (PDF – 233KB)
- February 20, 2019 – Celiac Disease (PDF – 200KB)
- December 4, 2018 – Fabry Disease (PDF – 171KB)
- October 23, 2018 – Gene Therapy as a Treatment Modality for Hemophilia (PDF- 205KB)
Patient-Led Listening Session Summaries
- January 27, 2020 – Ocular Melanoma (Summary coming soon)
- November 6, 2019 – Cerebral Cavernous Malformation (CCM)External Link Disclaimer
- September 17, 2019 – Osteogenesis Imperfecta (OI)External Link Disclaimer
- August 7, 2019 – Osteoarthritis (OA)External Link Disclaimer
- June 13, 2019 – Neurofibromatosis (NF) External Link Disclaimer
- May 29, 2019 – Fibrodysplasia Ossificans Progressiva (FOP)External Link Disclaimer
- January 16, 2019 – Amyotrophic Lateral Sclerosis (ALS) (summary unavailable)
- November 5, 2018 – Biliary Atresia, Progressive Familial Intrahepatic Cholestasis, Wilson’s Disease
PFMPD Webinars

Optimizing Digital Health Trials by Engaging Patients and Sites
01/12/2021 2:00 pm, Virtual, ZoomRegister now for this webinar on Jan. 12 at 2 p.m. Speakers include, Virginia Nido, Global Head, Product Development Industry Collaborations, Genentech; and Cindy Geoghegan, Patient Advocate.
Read More
Health Literacy Throughout Drug Development: Why it Matters to Pharma and to Patients
09/24/2020 3:00 pm, Zoom Read More
Patient-Focused Medical Product Development: Real-World Case Examples
08/13/2020 2:00 pm, Zoom Read More