Environmental Scan Information
Environmental Scan Considerations
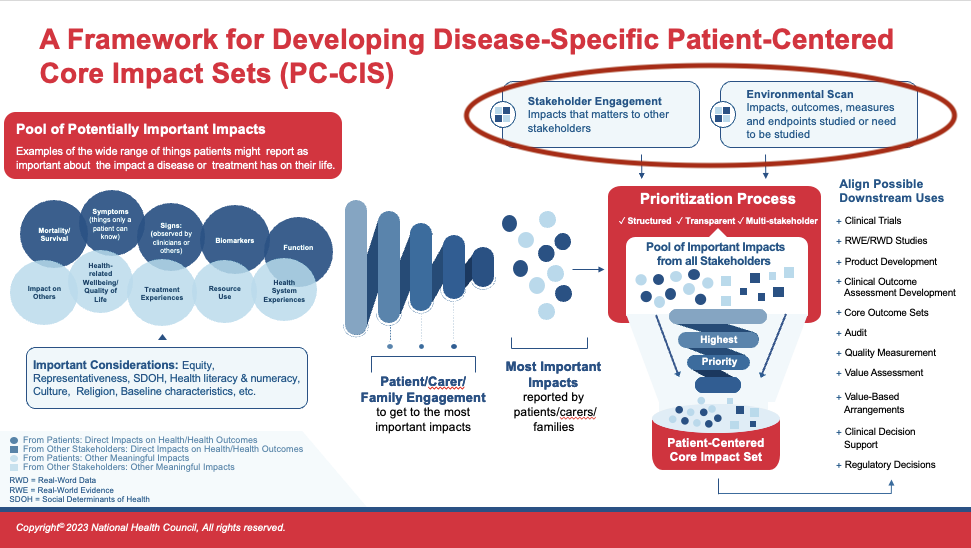
Even though Patient-Centered Core Impact Sets (PC-CIS) is led by and speaks largely to impacts identified as most important by patients, the input and experiences of other key stakeholders are also important to include. Hearing from and involving other stakeholders helps to make the PC-CIS optimally applicable and usable across a variety of contexts. Note, here we are using the term, “others,” in reference to clinicians and health care providers, researchers, payers, administrators, etc., and are excluding families and carers whose voices would have been captured alongside that of patients.
A logical place to begin this search is via existing literature, reviews, and an environmental scan. These compilations can exist in various forms including:
- Published reports, peer-reviewed and gray literature (non-traditional/non peer-reviewed), and government reports from sources like the Congressional Research Service
- Clinical practice guidelines
- Existing Core Outcome Sets (COS)
There may also be relevant, compiled insights from groups of stakeholders such as clinical professional societies, health technology assessment (HTA) bodies, or research entities in the form of position statements, formal recommendations, or guidances.
Similar to the evaluation of existing patient data about impacts, the use of other sources of existing data should trigger a review for validity and appropriateness of methodology. Additionally, there are tools for stakeholder mapping to ensure the full complement of inputs are being leveraged. A suite of tools and guidance available through the European Union’s Responsible Research & Innovation can be valuable as you evaluate the soundness and representativeness of your evidence base.
In evaluating the completeness of the “other-stakeholder data,” it is helpful to consider the types of stakeholders that are important to include. Depending on the population or disease state for which the PC-CIS is being developed, a variety of stakeholders may be important — including, but not limited to:
- Clinicians and other health care providers
- Researchers and clinical trial coordinators
- Health economists
- Purchasers or payers (i.e., employers and insurance companies)
- Biopharmaceutical, device, diagnostic, or life-science companies
- Educational institutions
Regulators and oversight bodies
Arthritis Use Case
Importantly, the pre-project ideation listening sessions, and then key informant interviews occurred before the literature review. Researchers helped identify the primary literature and landscape of what exists in the span of outcomes research for arthropathies but the existing literature, which might have biases, be outdated, or have neglected or over-ridden patient input did not drive the work.