Prioritization
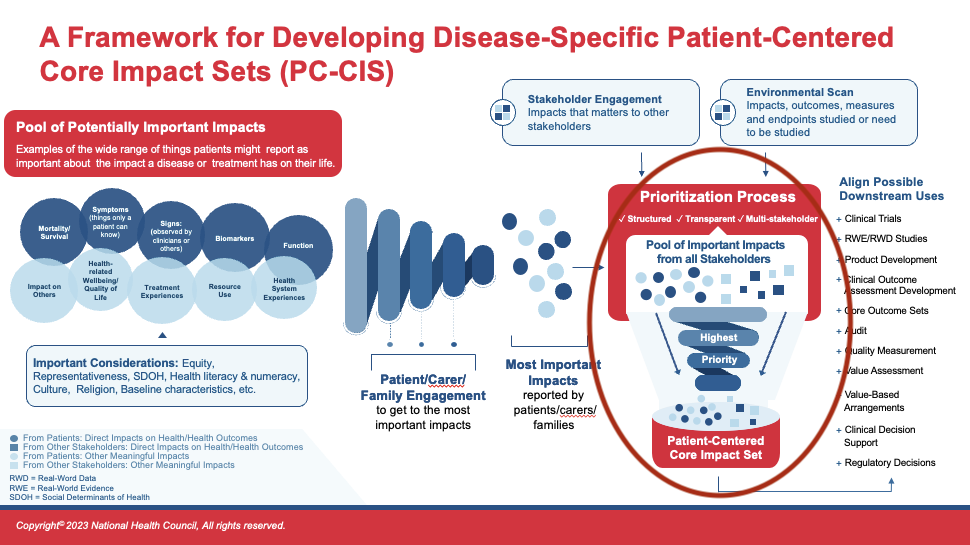
Prioritization of all Impacts
At this stage, the suite of patient-prioritized impact data is integrated with other stakeholder-informed impact data to be filtered through a second prioritization process of ALL impacts collected. It is expected that there will be at least some alignment between the two sources of data – but keep in mind that when there appear to be differences, it may be a difference in wording or description versus a true difference in content. Patients need to be heavily involved in ensuring their intended meanings are understood by the full group and impacts contributed by others are understood by patients.
By way of reminder, as PC-CIS are an emerging and evolving tool, there is no proposed goal for the “ideal” number of impacts to arrive at. The expectation is that the correct number of impacts will vary by condition or population. In the development of the PC-CIS, the goal should be to compile the number of impacts that is representative of the full body of issues most important to patients and families. There is no standard number.
Given the relativeness newness of this approach, (beginning with the collection and prioritization of patient data rather than other stakeholder data), patient organizations and patients leading and partnering in PC-CIS development must ensure other stakeholders are not able to out-vote (vote down) any impacts prioritized as important by patients. If patient-leaders need support during this stage, they can recommend other stakeholder partners review the sections of this Blueprint that articulate the importance of the order of these steps, and explicitly prevent the potential for patient-prioritized impacts to be discarded.
Ensuring the Patient Voice is Maintained
In this prioritization phase, the issue of particular importance is ensuring that impacts prioritized by patients cannot be disregarded. While most of the same tools and methods used for the first round of prioritization are equally applicable in this stage, there is one important step that is different. In the second round of prioritization, it is critical that impacts patients identify as important or worth keeping cannot be “voted out” of the final, prioritized list.
The group may want to consider a weighting structure that ensures patient-voted priority impacts are retained. While there are no current examples of PC-CIS creation methods (because they are still in the testing phase), there are several examples from multi-stakeholder Core Outcome Set (COS) development that are valuable resources to consider to maintain the patient voice:
- The Harmonizing Outcome Measures for Eczema (HOME) Roadmap for Core Outcome Sets Development
- The Center for Medical Technology and Policy (CMTP) COS Projects and methodology
- Patient and family engagement in the development of two rare disease COS
- CMPT coreHEM final report (consult page 6) provides guidance on ensuring patient-prioritized items are appropriately retained
- University of Maryland Patient-Driven Values in Healthcare Evaluation (PAVE) framework for prioritization
Arthritis Use Case
Again, the nominal group technique steps used for the INSIGHTS program provide an example for how PC-CIS co-creation could occur, with very intentional and repeated involvement of patients/families in each step, even during more complicated elements of the project. In this case, because the ultimate output was a tool to collect patient-reported outcomes in the community setting, there was less of a need to integrate “traditional” outcomes from literature and elsewhere, but the model is a solid basis for those seeking to build their own PC-CIS.
In the INSIGHTS program for both adult and juvenile disease, multiple in-person and virtual sessions were held with discussion and prioritization of key items, always informed by leadership from patient staff and patient/parent volunteers. This happened iteratively until a key set of items were finalized.